The provision of active sites that engender low-energy pathways for chemical reactions constitutes not only the foundation of the modern chemical industry but also the basis for the origin and sustenance of life on earth. Biocatalysts enable metabolic processes to occur at biologically relevant timescales just as synthetic catalysts render chemical processes viable at industrially relevant timescales. Our group is using metal-organic framework materials (MOFs)- an emerging class of catalytic materials- as a platform for mimicking enzymes from the standpoint of both active site structure and catalytic function. Insights gained from these studies are expected to lead to novel approaches to designing heterogeneous catalysts for industrial applications while addressing the longstanding gap between enzymatic and synthetic heterogeneous catalysis.
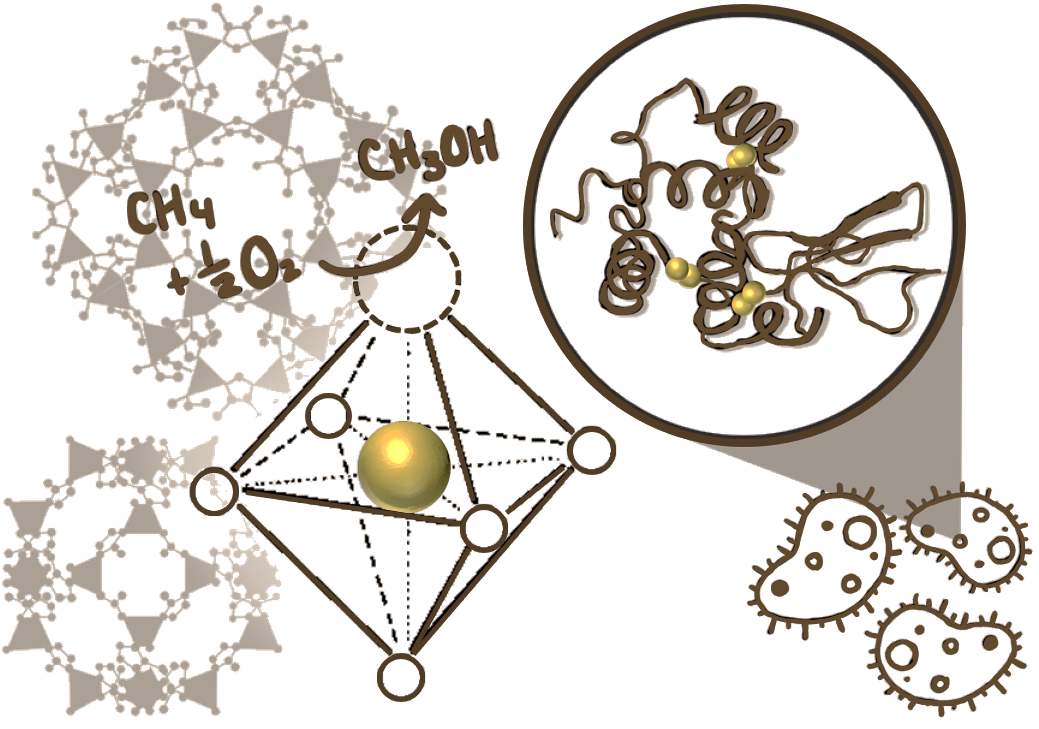
Catalytic Natural Gas and Biomass Conversion
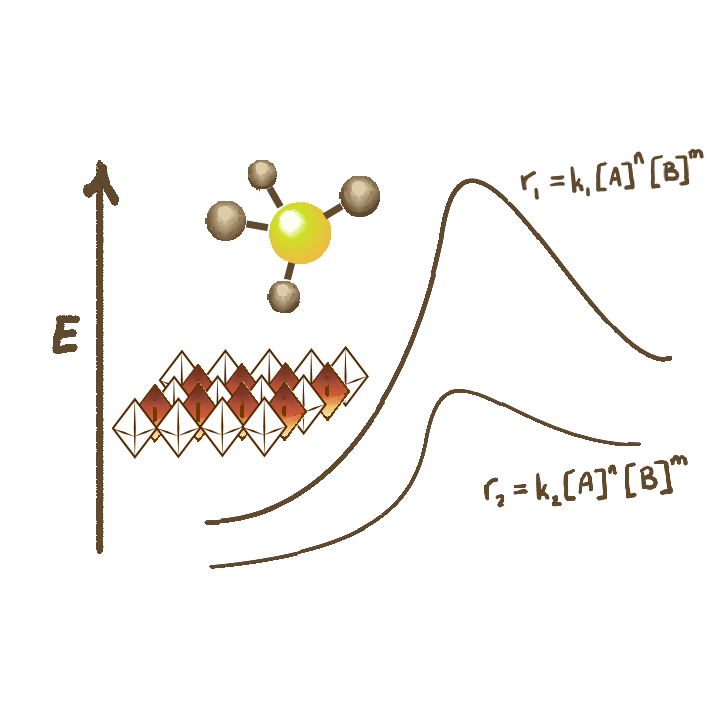
Bulk mixed metal oxide catalysts- widely used in large scale industrial applications- are significantly more complex compared to their zeolitic counterparts. As an example, in addition to the large variety of defect sites that their surfaces can host, several different facets can be exposed on a single catalyst material, and catalytic activity can originate from both amorphous and crystalline domains. This diversity in lattice defects, crystallinity, morphology and composition make elucidating active site requirements and reaction mechanisms highly challenging. Our group (in collaboration with the Rimer and Balakotaiah groups) is working towards using well-defined oxide crystals, steady state and transient kinetics, and non-isothermal reactor design to create and exploit a molecular-level understanding of natural gas and biomass conversion processes occurring over bulk mixed metal oxides.
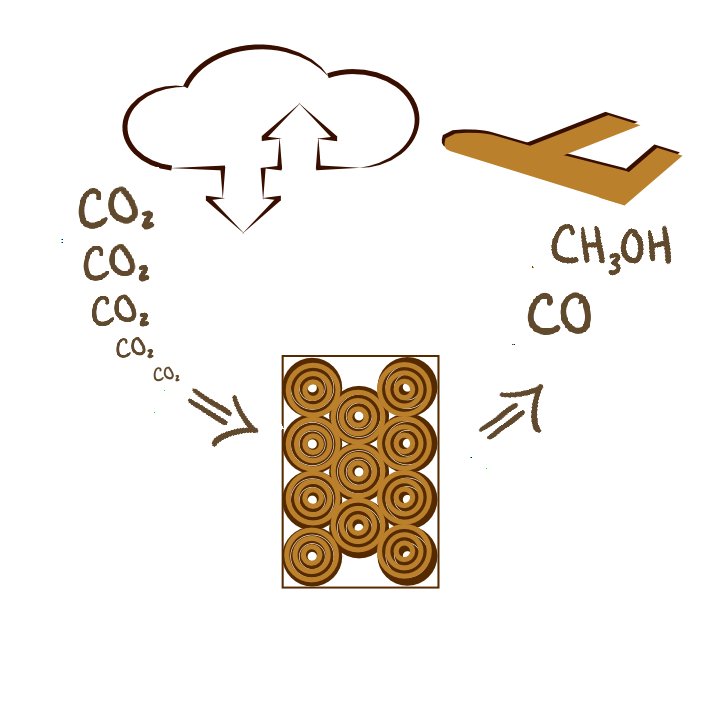
CO2 Capture and Conversion
Mitigating the adverse effects of anthropogenic CO2-induced climate change is one of the defining challenges of the twenty first century. Existing approaches to capturing CO2 from point sources and ambient air are expensive, creating an urgent need for low-cost capture and conversion technologies that are also scalable. Our group (in collaboration with the Krishnamoorti group) is working on understanding, at a molecular level, the mechanisms for capture and conversion of CO2 over nanoporous materials to valuable chemical products, and exploring contacting patterns and opportunities for integration into low-cost or negatively priced intermittent energy sources.
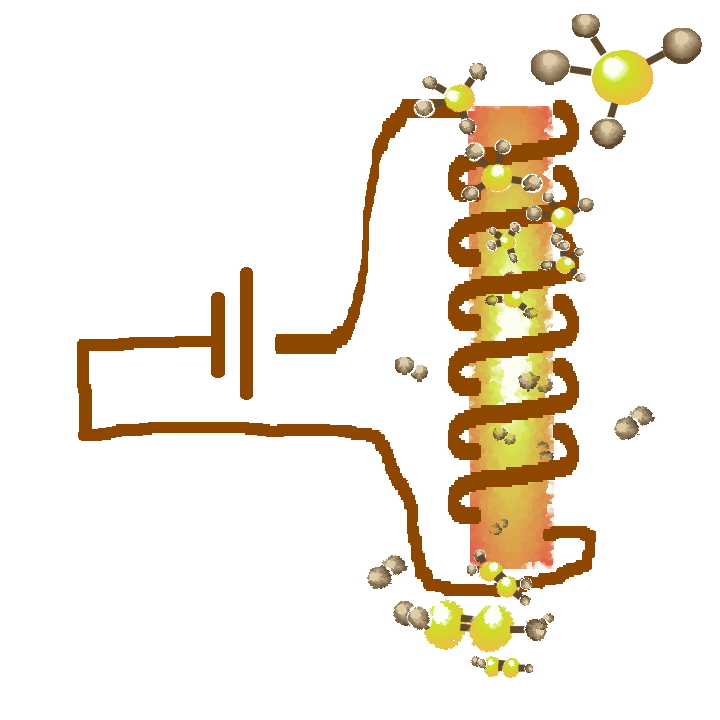
Electrified catalysts, reactors, and processes
Many of the largest volume chemicals produced today involve catalytic endothermic reaction steps. Most of these endothermic chemical processes are driven by energy generated from the combustion of fossil fuels including methane. Our group (in collaboration with the Balakotaiah and Harold groups) is developing catalyst and reactor configurations in which active sites are resistively heated by flowing a current through a conducting substrate. Electrical heating of catalysts will be enabled by the use of renewable sources of electricity that are becoming increasingly cost-competitive over time. The proposed catalyst and reactor technology will lower significantly the carbon and energy footprint of the chemical industry, and also help advance the distributed, modular manufacturing of value-added chemicals.